This webpage summarises the changes in immunoglobulin product supply in 2025.
Why is this happening?
The changes are due to the start of a new framework agreement between the NHS in England, Scotland, and Northern Ireland*, and IG suppliers and introduce a new IVIG product, Gamten, manufactured by Octapharma, from UK-sourced plasma.
*Wales is not part of the new framework, having its own contractual agreements with IG suppliers.
What are frameworks?
Frameworks are public contracts awarded following a competitive tendering procedure. The new IG framework will be in place from 1 April 2025 to 31 December 2027, with an option to extend for a period of up to 15 months. The framework will introduce changes in IG brand availability that will be applied across all medical specialities including immunology, haematology, neurology and infectious diseases.
What this means for you
You may not be affected by this change if the supplier listed below has been awarded a contract as part of the new framework. However, the implementation of the new 2025 framework does mean significant numbers of patients will have to change IG brands this year.
We have been informed that the NHS is working with nurses, clinicians, pharmacists, and home care delivery companies to ensure the transition works smoothly. Immunodeficiency UK understands you will be contacted by your clinical team if you are affected, with the timing of switching your IG product depending on your healthcare team’s capacity and the type of product you are on**. Patients on subcutaneous products have until the end of 2025 to switch if that is required, whereas intravenous therapy patients will switch sooner.
**Please note there may be differences between how different immunology centres in England, Scotland and Northern Ireland deal with the timing and logistics of switching patients onto different products.
All IG products are considered safe and equally effective, but change often introduces anxiety amongst patients, their carers or families. Immunodeficiency UK is committed to making sure patients have the necessary information to have informed discussions with their clinical teams.

Our leaflet on switching immunoglobulin (IG) products may help address your concerns about switching products while supporting clinical discussions concerning this process.
The changes
Immunodeficiency UK has received communications from NHS England concerning this process. We have added notes in parentheses [ ] to provide more context, and links to further information.
In 2025, there will be several changes to the supply of Immunoglobulin (IG) products aimed at increasing self-sufficiency and ensuring more reliable product availability.
New Immunoglobulin Framework (April 2025)
In April 2025, a new framework for the supply of IG will be launched, comprising three main suppliers to cover the demand for IG products. This framework streamlines and strengthens the supply chain while ensuring that patient needs are met efficiently and securely.
Introduction of New Brand: Gamten 10% IVIg (Q1 2025)
Alongside the new framework, a new IG product, Gamten 10% IVIg, will be introduced in early 2025. This product is manufactured using the same process as Octagam 10%, but using plasma donated within the UK. Using domestically sourced plasma to manufacture plasma-derived medicinal products will improve the resilience of the NHS supply chain and enable the health service to better withstand global disruption. This contract will be in effect until at least January 2030, (with the option to be extended for a further 24 months).
The following suppliers and brands have been awarded:
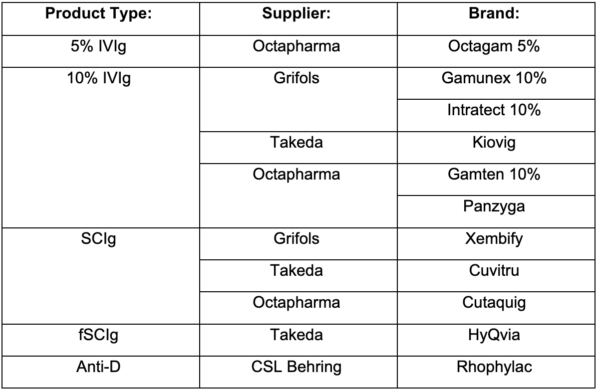
Changes to Product Supply: Patient Impact and Brand Switching
The new framework and awarded products will require some patients to switch to a different treatment brand.
There are no functionally significant differences in any product and patients and clinicians should be reassured accordingly.
If there is a clear clinical need that a patient cannot be treated with an awarded product, as above, your clinician can seek approval for the continuation of your existing medication.
[Immunodeficiency UK understands that the eligibility of clinical need and the approval process for continuation of existing treatment are undefined. Our current understanding is that in England your clinician will need to make an application to a Sub Regional Advisory Panel (SRIAP). SRIAPs were created by NHS England in 2018 to manage immunoglobulin usage by ensuring it is only used in accordance with national guidelines, as developed by an expert consensus group drawn from across the UK.]
The following suppliers and brands have not been awarded: [In previous NHS communications to us these were referred to as Reserve Suppliers. A Reserve Supplier does not have an assigned volume or confirmed share in the Framework agreement. However, NHS customers can purchase from this supplier to fulfil a defined clinical need or to address a shortfall in supply from one of the awarded suppliers].
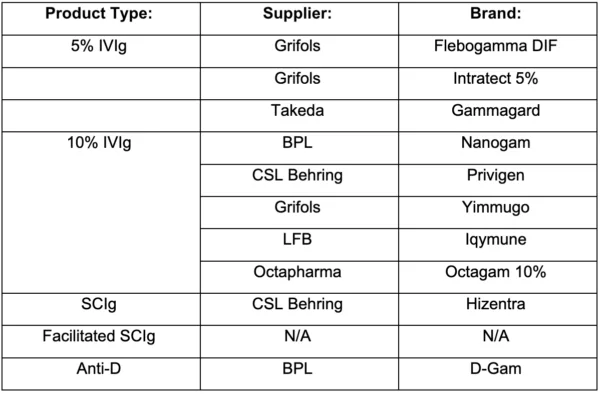
Implementation
Where a patient is identified as suitable for switching from their current brand, they will be contacted by a member of their clinical team. Patients’ treatment frequency and dosing will remain in line with national clinical guidelines [see link below] and patients will be monitored to ensure the product is well tolerated with no adverse clinical outcomes. It is anticipated that patients treated with IVIG will be switched by no later than September 2025, and patients on SCIG will be switched by no later than December 2025.
Patients identified for switching will be contacted by their clinical team from January 2025 onwards. Where possible, patients will be switched at their next scheduled appointment to minimise the need for additional visits.
Ends
Further information:
- Information on Frameworks can be found at Guidance: Frameworks (HTML) – GOV.UK.
- Information on the safety and impact of switching – Switching immunoglobulin products, what are the implications? Result of 2018 census of immunology centres.
On the collection of UK plasma and UK plasma-derived immunoglobulin product:
- NHS Blood and Transplant Service Update: Delivery of UK Plasma to be made into life-saving medicines. 11/9/24.
- Statement on the UK Plasma for Medicines Programme from NHS England’s Medicines Procurement and Supply Chain (MPSC) team. 21/8/24.
NHS Scotland resources:
Dated 4th February 2025; revised 20th February 2025





